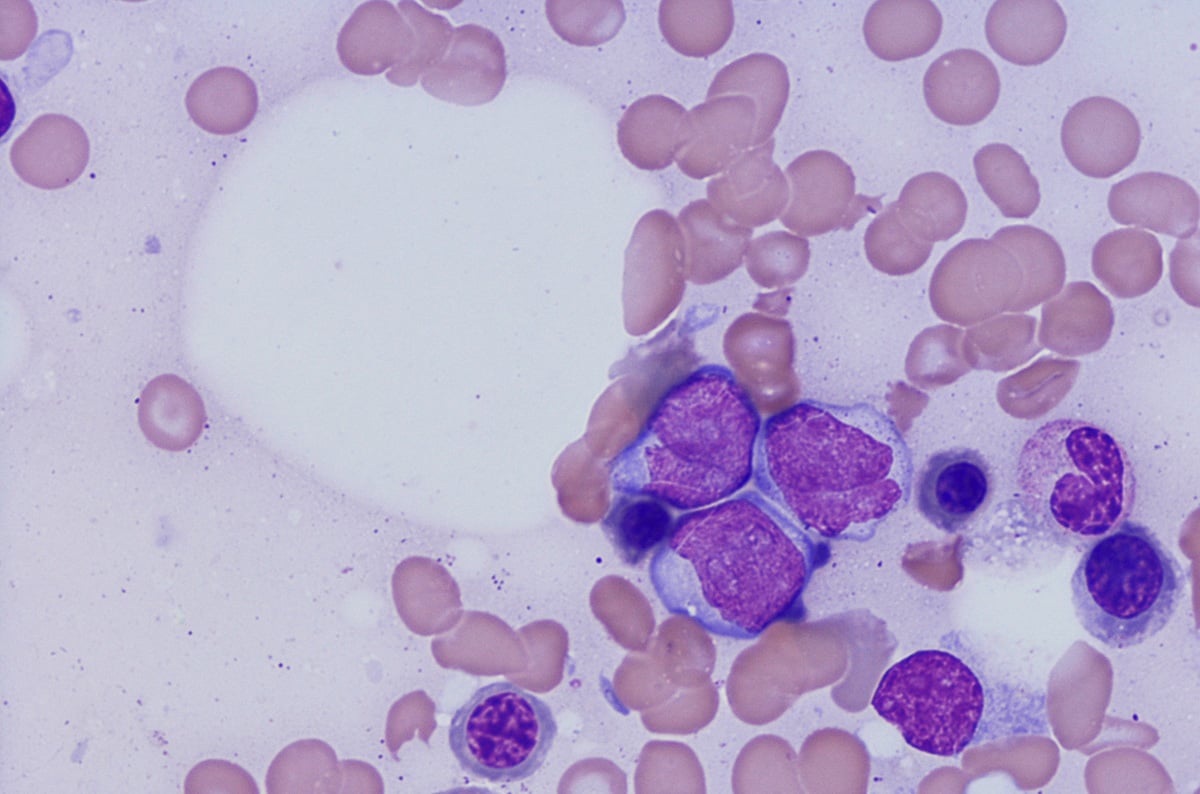
백혈병 및 림프종
Hematologic malignancies (blood cancers) are diseases of immune system cells and are traditionally categorized according to where the cancer is first detected; in the blood (leukemias),2 lymph nodes (Hodgkin and non-Hodgkin lymphomas)3 or bone marrow (myelomas).4

Cancer incidence and mortality are growing rapidly worldwide, and can be attributed to both the aging and the growth of the population, as well as changes in the prevalence and distribution of major risk factors.1 Blood cancers account for approximately 6% of all cancer cases, with close to 1.2 million new cases registered in 2020, and nearly 700,000 deaths.1 The growing burden of this group of diseases makes it increasingly important to effectively diagnose and characterize them early, to allow the best selection of treatment as soon as possible.
Types of blood cancer
Leukemias are derived from changes to blood cells that can occur at any time during their formation from hematopoietic stem cells by hematopoiesis. The exact point at which this transformation occurs gives rise to the specific type of leukemia,5 and this is why lineage identification is so important in classifying the exact condition. Leukemias can be lymphoid or myeloid – depending on the type of blood cell affected – and acute or chronic, depending on the rate of disease progression.2
Lymphomas result from malignant transformations in lymphocytes. There are a number of subtypes which are based on surface markers, tumor architecture, cell morphology and differentiation, and genetic alterations.6 Early identification of the lymphoma subtype is important to optimize treatment.

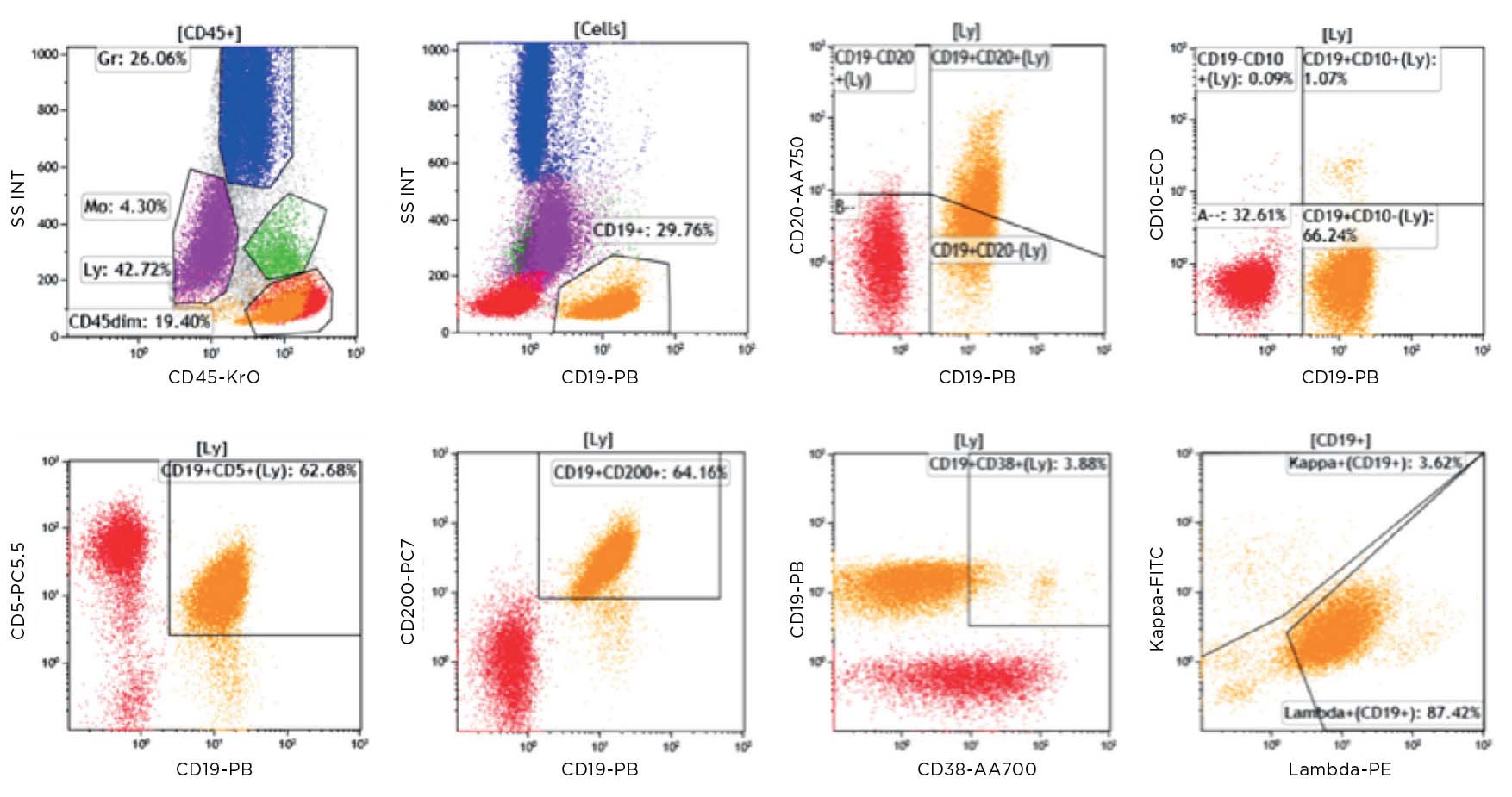
Flow cytometric immunophenotyping (FCI) is a useful tool for the diagnosis, classification, staging, and monitoring of hematologic malignancies. The technique relies on a panel of antibodies that detect markers on the cells, aiding the identification of cancerous cells and their lineage. The ability to show subtle differences in antigen density also allows this technique to distinguish and characterize aberrant cells that may be present in tiny quantities. Flow cytometry has several advantages over immunohistochemistry (IHC) – the method that has traditionally been used to diagnose blood cancers – and these include:
- Defining distinct cell populations by their size and granularity
- Identifying dead cells to remove them from the analysis
- Detecting weakly expressed surface antigens
- Measuring several intracellular or surface antigens simultaneously, using multicolor analysis
- Delivering results quickly
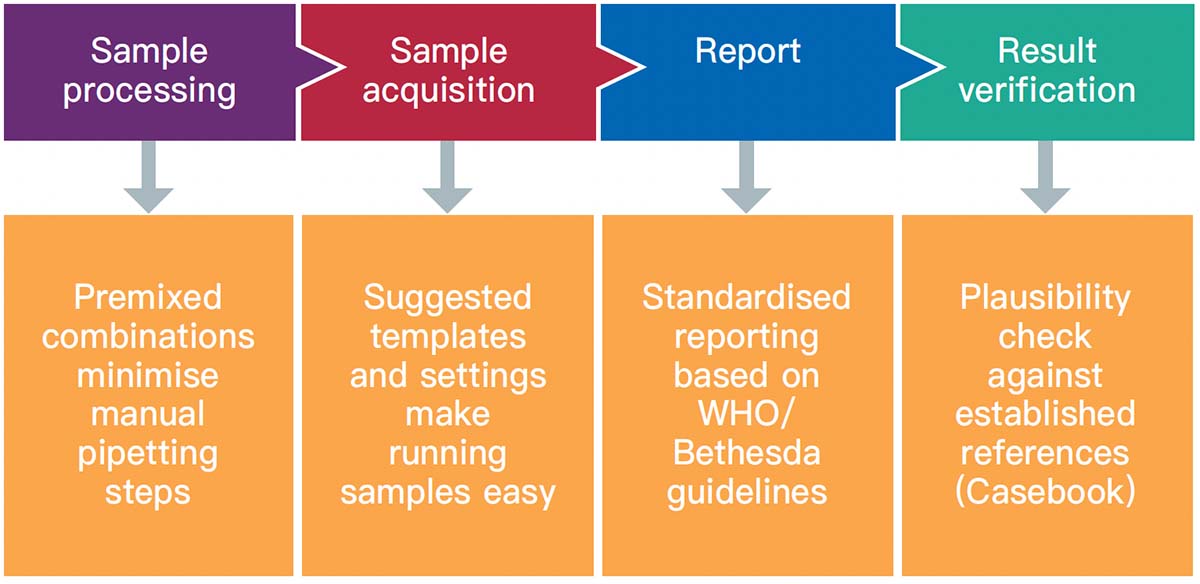
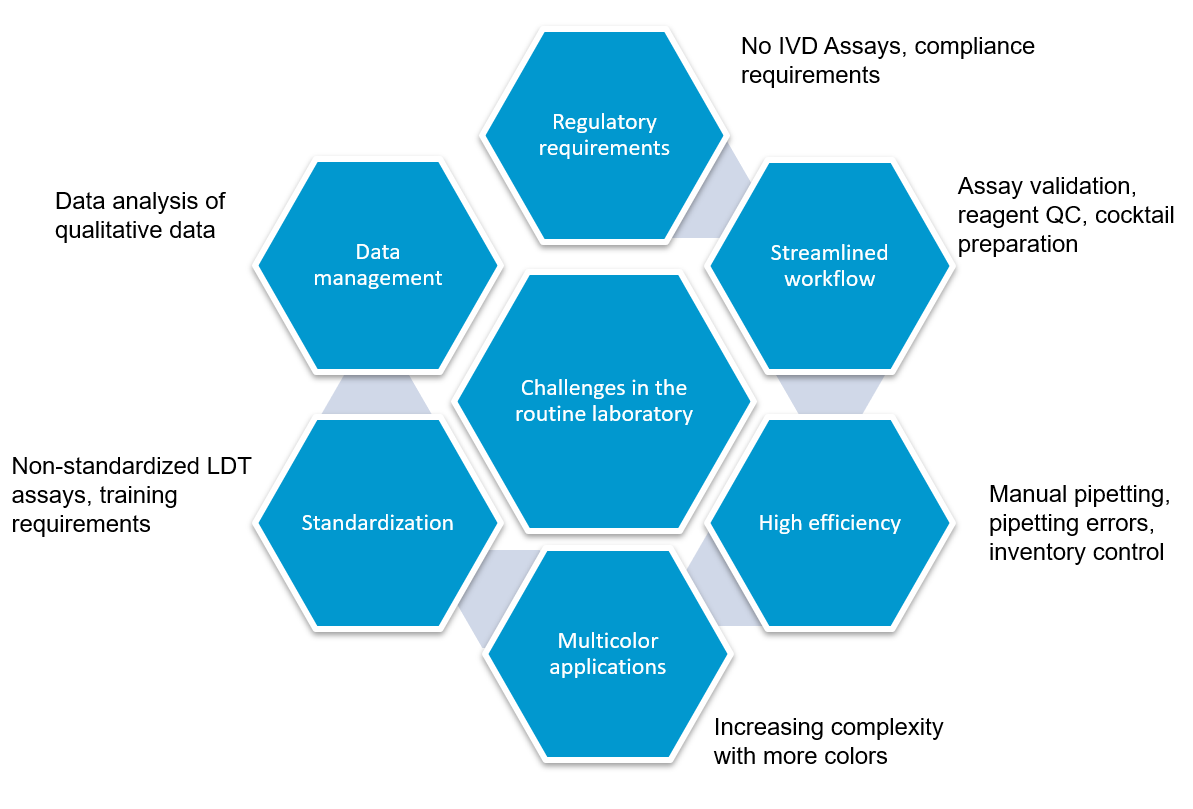
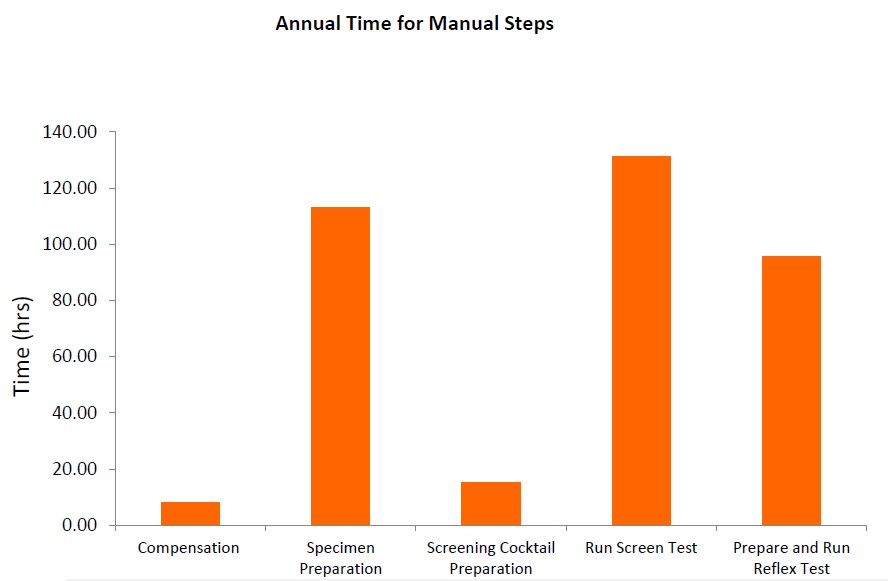
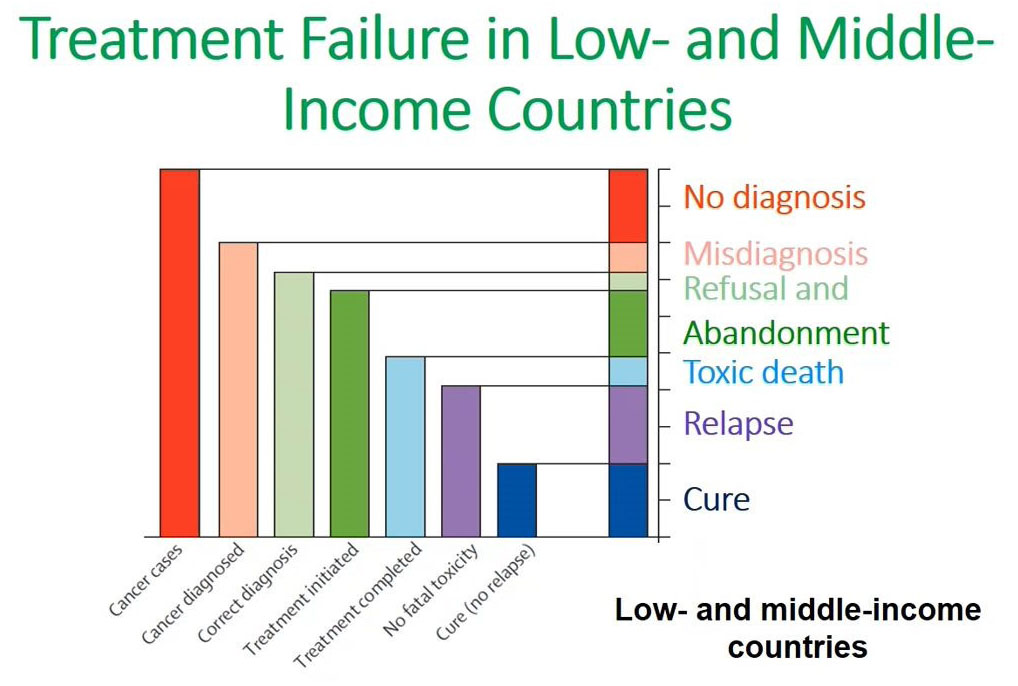
Flow cytometry immunophenotyping has not yet fulfilled its potential to fully support the clinical diagnosis of hematologic malignancies. The reliance on LDTs still provides several challenges to labs, that are summarized in the following graphic:
We are committed to support labs around the world to delivery accurate and timely results. We partner with you to support you in your patients' journey.
How to address regulatory requirements
How to increase workflow efficiency

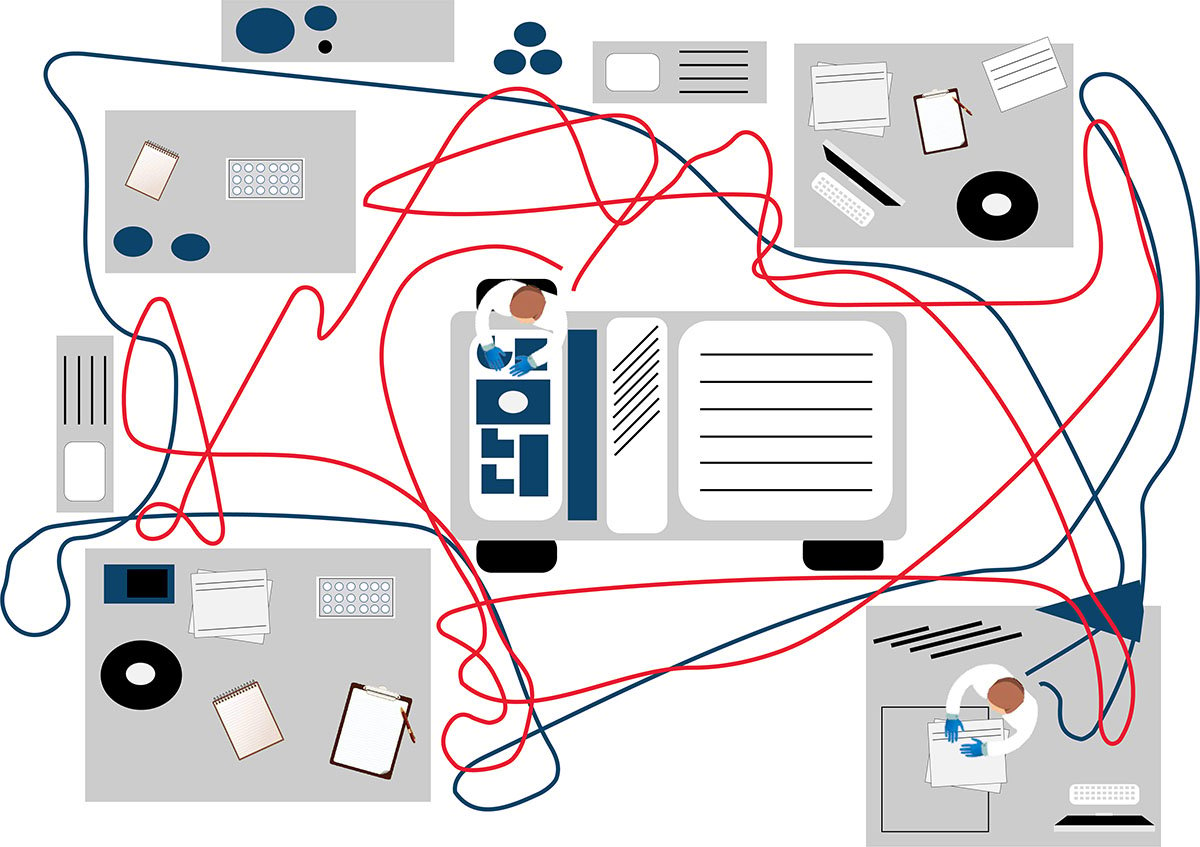
LDTs for FCI rely on the manual preparation of the antibody cocktail used in the screening panel. Any process involving manual pipetting is liable to errors, for example, because of mistakes made by the operator, or incorrect calibration of the pipettes. Furthermore, the use of ‘wet’ reagents means that there is a risk of them degrading over time. Standardized, dry, pre-mixed antibody panels eliminate the risk of incorrect preparation. In addition, by adopting a standardized test with built-in controls, labs can be confident in the results produced.
There is a high capital cost associated with the purchase of a flow cytometer, so ensuring the efficiency and accuracy of the tests being run provides maximum return on this investment.
The complexity of antibody preparation for LDTs can have significant cost implications as, in some labs, technicians are dedicated entirely to preparing the flow cytometry panels. Standardized reagents, however, can reduce manual input, waste, and errors, and can lead to considerable savings.
You are asked to do more with less? Learn how ClearLLab can help you save cost!
Often times, LDTs use manually prepared and designed panels, where each individual antibody must be prepared and then validated. Once this is complete, the cocktail must be prepared and then the antibodies revalidated in this combination. Further adjustments may also be required if the mixing of antibodies affects the validation. Rigorous documentation in compliance with local regulations must be maintained throughout for quality assurance, along with regular batch and stability testing. This represents a significant proportion of a workflow, especially when as many as 10 colors are being used in the most modern flow cytometers. Using standardized reagents not only helps to shorten workflows and enables faster results, but it also frees up technicians to focus on data analysis rather than repetitive manual tasks.
Until recently, clinical data sharing and education have been hindered by the complexity and variability of test set-up and results analysis. Although different laboratories may base their tests on the same consensus guidelines, the lack of a single standardized approach means that results are often not comparable between institutions. Implementing standardized reagents and procedures across the global arena would provide a framework for cooperation, data sharing and education, improving the quality of both clinical education and research. Standardized approaches ensure that results conform with international guidelines, removing confounding factors such as variability in the set-up of protocols, and the interpretation of results.
Finally it is about confidence in results - for you and for your patients. Be Accurate
Clinicians rely on the accuracy of results to make correct diagnoses and treatment decisions. Misinterpretation of samples and an incorrect clinical conclusion could have serious consequences for the patient. Standardized testing can strengthen confidence of clinicians in the results they are given, and the knowledge that the same conclusion would be reached wherever the analysis had been carried out, allowing them to provide high-quality patient care. For patients, quicker and more accurate laboratory testing offers them the benefit of starting on the correct treatment pathway as soon as possible, providing the chance of a good outcome.
Ready to check out the Casebook? Yes, take me there!
ClearLLab 10C Workflow
The premixed, dry ClearLLab 10C panels use the DURA Innovations Technology and reduce the possibility of human error posed by laboratory developed tests. They eliminate 14 steps and streamline five more, helping you turn samples around faster and deliver results more quickly.
ClearLLab 10C Design
ClearLLab 10C Components

*Non-Hodgkin lymphoma only
References
- Bray F, Ferlaym J et al. (2018), Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68: 394-424. doi:10.3322/caac.21492
- American Society of Hematology. Leukemia (n.d.). https://www.hematology.org/education/patients/blood-cancers/leukemia Accessed December 02,2021
- American Society of Hematology. Lymphoma (n.d.). https://www.hematology.org/education/patients/blood-cancers/lymphoma Accessed December 02,2021
- American Society of Hematology. Myeloma (n.d.). https://www.hematology.org/education/patients/blood-cancers/myeloma Accessed December 02,2021
- Arber DA, Orazi A et al. (2016), The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127(20); 2391-2405. doi: 10.1182/blood-2016-03-643544 https://ashpublications.org/blood/article/127/20/2391/35255/The-2016-revision-to-the-World-Health-Organization
- Swerdlow SH, Campo E et al. (2016), The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 127(20): 2375-2390. doi: 10.1182/blood-2016-01-643569 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4874220/