Preparation of Mouse Plasma Microsamples for LC-MS/MS Analysis Using the Echo Liquid Handler
Randy DyerIntroduction
Serial microsampling of animals reduces the overall number of animals sampled and euthanized for toxicology studies. Traditional composite studies in mice can require up to 700 μL of blood for each time point in a pharmacokinetic study. A draw of 700 μL from a mouse would require the animal to be euthanized and often require the use of addition “satellite” animals to complete the study. Thus, in a 9-point time course analysis of mouse plasma, nine mice would be sacrificed.
In response to programs like the EU 3Rs initiative to reduce the use of animals in research researchers have turned to microsampling as an alternative to composite studies. With advances in bioanalytical techniques drug levels can be determined from samples of 20 μL or less. This small sample volume or microsample enables reduces the dependency on satellite animals to complete a study. Additionally, microsampling is faster and less stressful than traditional composite studies.
The adoption of microsampling for toxicology studies has been slow and methods using enabling technologies like Echo acoustic liquid handling have not been published. To help guide researchers interested in using Echo Liquid Handlers for microsampling, we developed this document that describes a method used by researchers at Hoffman-La Roche AG.
Step 1
Dosing the Mice
Animals are treated with compounds dissolved in a formulation buffer in two different ways depending on the type of study:
- Intravenous (IV) typically with a dose of 1 ng/kg
- Per os (PO) typically with a dose of 3 ng/kg
Once a mouse is dosed, blood samples are drawn for an 8-point time course analysis.
Step 2
Blood Sample Collection
Blood is collected from the mouse at eight time points:
1. The animal cages are placed in a warmed environment (heat lamp).
2. After warming the mouse tail in a water bath (approx. 50°C), mice are kept in a restrainer for blood sampling.
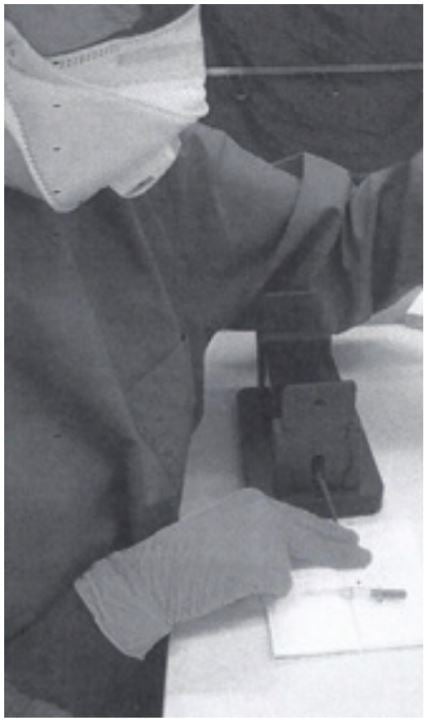
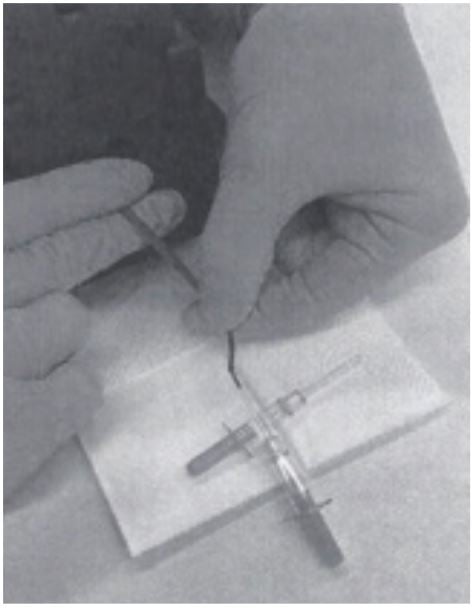
3. The tail vein is punched with a cannula (outer diameter 0.6 mm) and blood is collected directly into a Minivette POCT 20µl K3E, which is EDTA treated and has a sample pickup volume of 20 μl (Sarstedt cat. no. 17.2113.020). The tail of the mouse has to be massaged to support blood flow, if necessary.
4. Once the Minivette is filled, blood is immediately transferred into an Eppendorf PCR Tube (0.2 ml, Eppendorf cat.no. 0030124332). (The blood can be dispensed into the tube by using the plunger of the Minivette.
5. After blood collection a gentle pressure is applied to the vein to stop further bleeding.
6. The tube with the blood is loaded into an Eppendorf Centrifuge 5424R and spun down at 4°C for 5 min with 11,000 rpm.
7. The blood separates into the three layers and after centrifugation 8 µl of plasma are aspirated from the top plasma layer using a pipette making sure not to disturb or aspirate the buffy coat layer underneath. The plasma is then transferred into a fresh Eppendorf PCR Tube and stored at -20C° until further sample preparation.
Step 3
Preparation of Samples for Transfer Using the Echo Liquid Handler
The plasma samples of the treated animals (unknown plasma samples) are taken from the freezer and the 8 μL of plasma are transferred into an Echo-qualified 384 LDV microplate (LP-0200). After the samples are transferred they are sealed with adhesive seal immediately to prevent evaporation. In addition to the unknown plasma samples, other wells of the same microplate are filled with blank plasma samples, pure DMSO, the analytic compound and an internal standard (Bosentan at 200 μg/ml in DMSO) to create a standard curve at different concentrations in DMSO:
- 1000 µg/ml
- 500 µg/ml
- 50 µg/ml
- 5 µg/ml
- 0.5 µg/ml
- 0.05 µg/ml
The transfer of reagents into the 384 LDV microplate is done with a manual pipette.
Step 4
Transfer on the Echo Liquid Handler
The contents of the 384 LDV microplate are transferred using the Echo system to an ABgene Storage plate (384-well, 55 µL, round well, V-bottomed, ThermoFisher Scientific, cat.no. AB-1056).
Using the compound concentrations provided in Step 3, a protocol to assemble a standard curve is created in the Echo Dose Response Software Application using the following parameters:
- 14 dilution points
- 2-fold dilutions
- Top concentration: 15,000 ng/ml
- 2% DMSO max. (10 nl)
- Automatic backfill
Running the Echo Dose Response protocol on the Echo system produces a 14-point dilution of the analytic compound in the ABgene plate. In a subsequent run on the Echo system 490 nL of blank plasma is transferred to each well of the standard curve and 500 nl of each unknown plasma sample is transferred to adjacent wells of the ABgene plate. This is followed by a transfer of 12.5 nL of the internal standard to each well of the standard curve and to each well containing unknown plasma sample.
The samples are now prepared for downstream assays.
Step 5
Downstream Sample Preparation
For protein precipitation 30 µL of MeOH is added into the wells of the ABgene plate using bulk reagent dispenser (ThermoFisher Multidrop Combi for example). The plate is sealed with an adhesive seal. This corresponds to a 60x dilution, which is high compared to a traditional workflow (usually 3x). Matrix effects in the mass spec analysis coming from traces of formulation buffers in the samples (from dosing) are eliminated at the higher dilution level. The level of detection is obviously also reduced by the dilution, but that’s typically not critical using the highly sensitive mass spectrometers.
Step 6
LC-MS / MS Analysis
At Roche they use a Shimadzu UFLC system for the liquid chromatography. The mass spectrometry analysis is done on an AB SCIEX QTRAP 5500 or 6500+ system. The sealed plate is loaded into the cassette of the injector module, which automatically pierces the seal with a needle. Depending on the compound 1–10 µL of a sample is injected every 3 minutes. The mobile phases are:
- Mobile Phase A: 90% H2O + 10% ACN + 0.2% HCOOH
- Mobile Phase B: 100% ACN + 0.2% HCOOH
- Mobile Phase C: 90% H2O + 10% MeOH + % HCOOH (rarely used)
The flow rate can range between 1.4 to 1.5 ml/min.